Nitrates - Pharmacology and Clinical Uses
Organic nitrates and nitrites have been used in the treatment of angina for well over 100 years. In 1857, inhalation of amyl nitrite, a volatile liquid and known vasodilator, was found to relieve anginal pain; however, the duration of action was brief and the dosage difficult to control. Organic nitrates were soon discovered to share many of the pharmacological properties of amyl nitrite, and by 1879 the sublingual administration of nitroglycerin was established for relief of acute anginal attacks. Research during the 1970s and 1980s established that nitrates and nitrites act via the formation of the reactive free radical, nitric oxide (NO). Thus, the term nitrovasodilator was coined to describe those nitrates, nitrites, and other compounds that are denitrated to release nitric oxide.
Endogenous Nitric Oxide:
Studies in isolated blood vessels demonstrated that acetylcholine acts upon endothelial cells to release a diffusible vasodilating substance whose chemical identity was unknown. The unidentified mediator was initially named "endothelium-derived relaxing factor (EDRF)" because of its inhibitory effect on vascular smooth muscle. Research by several laboratories noted striking similarities between the pharmacology of the nitrovasodilators and EDRF, thus leading to the proposal that EDRF is identical to NO. The release of NO from endothelial cells was subsequently confirmed and the importance of NO as a signaling molecule in the cardiovascular system, as well as other systems throughout the body, is now well established. In the cardiovascular system, endothelium-derived NO plays a key role in the local control of blood flow, regulation of blood pressure, and prevention of platelet aggregation and adhesion. Moreover, impaired NO signaling (e.g. decreased NO synthesis, release, or bioactivity) is associated with a number of common cardiovascular disorders (e.g. atherosclerosis, hypertension, diabetes, etc.). In 1998, the Nobel Prize was awarded to Furchgott, Ignarro, and Murad for their work on EDRF/NO-signaling in the cardiovascular system and elsewhere.
The interaction of agonists with endothelial cell receptors (R) leads to activation of nitric oxide synthase (NOS), resulting in the formation of nitric oxide (NO) from l-arginine. NO is also formed directly from exogenous nitrovasodilators, such as nitroglycerin (NTG), isosorbide dinitrate (ISDN), and isosorbide mononitrate (ISMN). NO activates the soluble form of guanylyl cyclase (GC), which catalyzes the formation of cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). Increased levels of cGMP cause relaxation of vascular smooth muscle. Phosphodiesterase (PDE) hydrolyzes cGMP to GMP.
Recent evidence suggests that NO may also activate, either directly or through cGMP-dependent mechanisms, potassium channels on the smooth muscle cell surface. The efflux of potassium ions hyperpolarizes the cell membrane, resulting in vascular smooth muscle relaxation.
Pharmacokinetics:
- Nitroglycerin, isosorbide dinitrate, and isosorbide 5- mononitrate, an active metabolite of isosorbide dinitrate, are the nitrovasodilators most commonly used in the clinical setting.
- All are lipid soluble and readily absorbed via several routes of administration.
- Most organic nitrates have very low oral bioavailability due to the presence of high-capacity nitrate reductase enzymes in the liver (exception is isosorbide mononitrate, has nearly 100 percent bioavailability following oral administration).
- Most denitrated metabolites are glucuronidated and excreted via the kidney.
- Nitroglycerin may be administered via several routes, including sublingual, buccal, oral, transdermal, and intravenous.
- Because of its rapid onset (1-3 minutes) and avoidance of first-pass hepatic metabolism, the sublingual route is preferred.
- Since nitroglycerin is moderately volatile and adsorbs to plastic, the sublingual tablets must be stored in tightly closed glass containers.
- The duration of action of sublingual nitroglycerin is relatively brief (~30 minutes).
- Nitroglycerin is readily absorbed across the skin and may be applied as either an ointment or transdermal patch.
- Intravenous nitroglycerin is used to rapidly attain therapeutic blood levels.
- The concentration can be quickly and safely titrated to the desired level, and the hemodynamic effects can be terminated rapidly by stopping the infusion.
- Isosorbide dinitrate and isosorbide mononitrate are primarily administered via the oral route.
Hemodynamic Effects:
- Myocardial Oxygen Demand: Nitroglycerin causes relaxation of vascular smooth muscle in both arteries and veins, although the effect on veins predominates at low doses. By dilating veins, nitroglycerin increases venous capacitance and decreases venous return to the heart - decreases preload (as described by the Laplace relationship). Arterial dilation by nitroglycerin decreases peripheral vascular resistance and leads to a reduction in afterload. Reduced preload and afterload result in decreased left ventricular wall tension, a major determinant of myocardial oxygen demand. Thus, the anti-ischemic effects of the nitrovasodilators are largely due to their ability to decrease myocardial work and oxygen consumption.
- Myocardial Oxygen supply: The nitrovasodilators have several effects on the coronary circulation, including dilation of large and intermediate-size coronary arteries, increased collateral flow, and redistribution of flow to ischemic regions of the heart. Accordingly, these beneficial effects are primarily responsible for the ability of nitrovasodilators to improve myocardial oxygen supply.
Clinical Use:
- Nitrovasodilators are used in the treatment of most forms of angina. Patients with chronic, stable angina often have fixed atherosclerotic lesions that obstruct blood flow in the large coronary arteries. The beneficial effects of nitrovasodilators in stable angina are due primarily to their ability to decrease myocardial oxygen demand. By decreasing preload and afterload, nitrovasodilators reduce ventricular wall tension and myocardial oxygen consumption.
- In patients with variant (Prinzmetal's) angina, the major underlying cause of angina is vasospasm of one or more coronary arteries. Intense vasoconstriction decreases coronary blood flow, thereby reducing myocardial oxygen supply.By dilating constricted coronary arteries and restoring coronary blood flow, nitrovasodilators increase myocardial oxygen supply and relieve variant angina.
- Nitrovasodilators are also used in treating patients with unstable angina. The pathophysiology of this condition is often complex and may involve several underlying factors superimposed on one another, including rupture of atherosclerotic plaques and thrombus formation, constriction of coronary arteries, and increased myocardial oxygen demand. In these patients, the beneficial effects of the nitrovasodilators are likely due to both dilation of the coronary arteries and a reduction in myocardial oxygen consumption.
- Nitrovasodilators are used for the immediate treatment of acute angina, as well as for long-term prevention. Because of its rapid onset of effect, sublingual nitroglycerin is the agent most frequently used to terminate an acute attack of angina. It may be used prophylactically when administered immediately prior to activities known to precipitate an anginal attack (e.g. physical exertion).
- Intravenous nitroglycerin is also used to control acute angina.
- Transdermal nitroglycerin and oral formulations of nitroglycerin, isosorbide dinitrate, and isosorbide mononitrate are widely used in long-term maintenance therapy of angina. Although sustained plasma drug levels can usually be attained with these products, their clinical efficacy may be limited by the development of tolerance.
Adverse Effects and Precautions:
The most frequently observed adverse effects of the nitrates are a direct result of the vasodilation produced by these drugs. Headache, due to dilation of cranial blood vessels, is common and may be severe. Symptoms of postural hypotension (e.g. dizziness) may also be encountered. Paradoxically, nitrates may increase myocardial oxygen demand in some patients by causing reflex tachycardia.
Concurrent use of nitrates and sildenafil may cause a sudden and dramatic drop in blood pressure, prompting the FDA to issue a warning about prescribing sildenafil to patients being treated with nitrovasodilators.
Although rarely used today as a therapeutic agent, amyl nitrite has gained popularity as a recreational drug. Inhalation of amyl nitrite, as well as isobutyl nitrite, purportedly enhances sexual pleasure and produces euphoria. Abuse of these products may cause severe cardiovascular toxicity.
Nitrate Tolerance:
Continuous or repeated exposure to high doses of organic nitrates rapidly leads to a reduction in the hemodynamic and antianginal effects of these drugs. This phenomenon, known as nitrate tolerance, may occur within 24-48 hours following exposure and represents a major limitation to the therapeutic use of organic nitrates. The cause of nitrate tolerance is unclear but it is likely to be multifactorial. Several mechanisms have been proposed including: (i) decreased activation or desensitization of the NO/cGMP signaling pathway (including reduced bioconversion of nitrates to NO); (ii) expansion of plasma volume; (iii) increased release and/or sensitivity to endogenous vasoconstrictors, such as endothelin, angiotensin II, and catecholamines; and (iv) increased production of superoxide anions, which destroy NO.
The most widely accepted method for preventing nitrate tolerance is to provide a period of low nitrate exposure during each day. For example, a common dosing strategy for transdermal nitroglycerin is to apply the patches for 12 hours and remove them for 12 hours each day.
Oral Drug Delivery
Most drugs are absorbed from the gastrointestinal tract and because of the convenience, orally administered drugs are widely used over injectable preparations. Some drugs like benzylpenicillin or insulin are destroyed by the acid and enzymes in the gut and therefore have to be administered parenterally.
Drug Absorption At The Small Intestine
Little absorption occurs before the drug reaches the small intestine. This is because when exposed to the gastric pH, basic drugs are ionised and therefore pass into the more alkaline small bowel before being absorbed. More importantly, most absorption occurs here simply because of it’s massive surface area.Most drugs are absorbed by passive lipid diffusion though there is a degree of carrier-mediated transport. Levodopa, a drug used in Parkinson’s Disease, is transported by the carrier mechanism usually used for phenylalanine. Fluoruracil is a cytotoxic drug that employs the natural pyrimidine transport system. Iron is transported via specific carriers in the jejunal mucosa and calcium by a vitamin D-dependent carrier system. The rate of transfer is determined by the ionisation state and lipid solubility of the drugs. Strong bases with a pKa of 10 or more or acids with a pKa of less than 3 are fully absorbed because they are fully ionised.
Factors Affecting GI Absorption
It usually takes 1-3 hours for 75% of an ingested drug to be absorbed. There are several factors which alter this, both physiological and relating to drug formulation.
GI motility has a large effect on the rate of drug absorption. Gastric stasis, which occurs in migraine and diabetic neuropathy, slows drug absorption. However rapid gut movement can also impair absorption. Some drugs have an effect of absorption, metoclopramide is given in migraine to increase GI motility and increase absorption of analgesics. Muscarinic receptor blockers decrease motility.
After a meal, drugs are more slowly absorbed simply because progress to the small bowel is delayed by the presence of food. Exceptions to this are propanolol which has higher concentrations after a meal because of the fact that food increases splanchnic blood flow. Clearly a decrease in splanchnic blood flow, for example in hypovolaemia, slows absorption. Drug particle size also plays a part. It has been observed in the past that different formulations of digoxin resulted in different plasma concentrations despite the fact that digoxin content in the tablets was the same. This was as a result of varying particle size.
Therapeutic drugs are formulated pharmaceutically to produce desired absorption characteristics. Capsules are designed to remain intact for several hours after ingestion with the aim of delaying absorption. Tablets with a resistant coating work on the same principle. Some drug formulations have a mix of slow and fast release in a capsule to achieve rapid and sustained drug action. By manipulating absorption rates, dose intervals can be increased and adverse side effects, associated with high peak plasma levels, reduced.
The physicochemical properties of drugs also play a part in drug absorption. Tetracycline binds to calcium ions and calcium-rich foods, delaying absorption. Bile acid resins such as colestyramine, bind warfarin and thyroxine.
Absorption is also decreased by both increasing age and disease.
Usually drugs that are administered by mouth, pass through the gut wall to the liver in the portal circulation and then into the systemic circulation where they can go about exerting their effects. However some drugs taken by mouth are designed to remain within the gut lumen until they are excreted. An example of this is the antibiotic vancomycin which acts within the gut lumen to eradicate Clostridium Difficile.
Bioavailability
Bioavailability is loosely defined as the proportion of drug that passes into the systemic circulation after oral administration, taking into account both absorption and local metabolic degradation. This is affected not only by the drug characteristics but also variations in enzyme activity in the gut wall and liver, gastric pH and intestinal motility.First-Pass Metabolism
First-pass or presystemic metabolism is a process occurring in the liver and even in the gut wall which extracts and metabolises some drugs so efficiently that the amount reaching the systemic circulation for the first time is considerably less than the amount absorbed from the gut. This means that a much larger dose is needed when a drug is taken orally than intravenously (when it is 100% bioavailable to the systemic circulation from the moment of injection). There is also an element of variability between individuals in the extent of first-pass metabolism of a given drug which leads to unpredictability when such drugs are taken orally. A drug that shows rapid first-pass hepatic metabolism has poor oral bioavailability.
Drugs in Pregnancy and Lactation
During pregnancy the mother and the fetus form a non-separable functional unit. Maternal well-being is an absolute prerequisite for the optimal functioning and development of both parts of this unit. Consequently, it is important to treat the mother whenever needed while protecting the unborn to the greatest possible extent. Drugs can have harmful effects on the fetus at any time during pregnancy. It is important to remember this when prescribing for a woman of childbearing age. The past use of stilboesterol in pregnant women with threatened abortion has resulted in development of adenocarcinoma of vagina in female children in their teens and early 20s. However, irrational fear of using drugs during pregnancy can also result in harm. This includes untreated illness, impaired maternal compliance, suboptimal treatment and treatment failures.
Such approaches may impose risk to maternal well-being, and may also affect the unborn child. It is important to know the ‘background risk’ in the context of the prevalence of drug-induced adverse pregnancy outcomes. Major congenital malformations occur in 2–4% of all live births. Up to 15% of all diagnosed pregnancies will result in fetal loss. The cause of these adverse pregnancy outcomes is understood in only a minority of the incidents.
Prescribing in Pregnancy:
In the placenta maternal blood is separated from fetal blood by a cellular membrane. Drugs can cross the placenta by active transport or by passive diffusion down the concentration gradient, and it is the latter which is usually involved in drug transfer. Placental function is also modified by changes in blood flow, and drug which reduce placental blood flow can reduce birth weight. This may be the mechanism which causes the small reduction in birth weight following treatment of the mother with b-blockers in pregnancy. Early in embryonic development, exogenous substances accumulate in the neuroectoderm. The blood-brain barrier to diffusion is not developed until the second half of pregnancy, and the susceptibility of the central nervous system (CNS) to developmental toxins may be partly related to this. The human placenta possesses multiple enzymes that are primarily involved with endogenous steroid metabolism but may also contribute to drug metabolism and clearance.
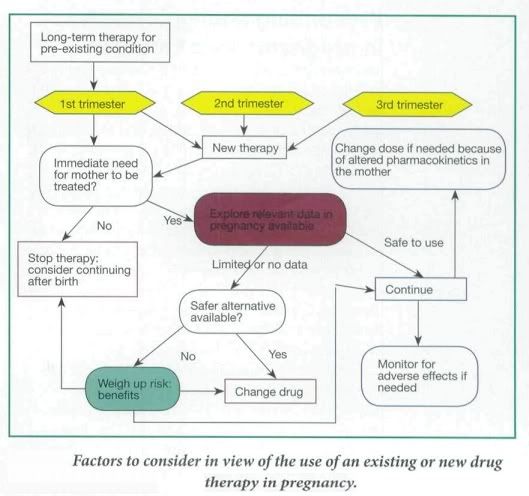
Factors to Consider
In general, most women of child-bearing age are young, fit and healthy. However in some cases the mother is on an established therapy for a pre-existing condition, such as depression or epilepsy, or she may develop a pregnancy-induced medical condition, such as hypertension or gestational diabetes. Therefore the aim of drug therapy is to limit any damage to the growing fetus while still being able to treat the mother appropriately. Ideally a detailed risk:benefit assessment of drug therapy in pregnancy should be conducted.
- The trimester in which the drug is being taken,
- What dose of drug is being taken,
- If the drug is at the lowest effective dose possible,
- How much is being passed through the placenta to the fetus and, consequently,
- If the drug is known to be teratogenic
- Total body water and plasma volume increase during pregnancy. In addition, the concentration of plasma albumin falls, reducing protein binding of drug and consequently increasing drug plasma levels. Hence doses of highly protein-bound drugs might need to be reduced (Phenytoin, Warfarin etc.)
- Pharmacokinetics of drug metabolism may differ in a woman during pregnancy and consequently this would need to be taken in to account during drug dosing. ( maternal underactive thyroid gland that has previously been controlled by taking levothyroxine at a particular dose may well need to be increased)
- Drugs taken during pregnancy can act directly on the fetus causing spontaneous abortion, fetal malformations, or have no effect.
- Drugs can also cause oxidative stress which can result in underdevelopment and malnutrition.
- Usually very early in pregnancy, before the 20th day, there is an all-or-nothing effect whereby the fetus will abort spontaneously if the drug has caused harm, or there will be no detrimental effects to the developing fetus (Mother is usually unaware).
- Between days 21 and 56 {approximately), following fertilization, the fetus is very susceptible to birth defects and this is when the mother has usually just realized that she is pregnant. This is the stage of organogenesis and drugs used at this stage may interfere in their development and cause a fetal malformation, or the defect may be more subtle and may not affect function to a debilitating extent or may even lead to a miscarriage.
The stage of gestation influences the effects of drugs on the fetus. It is convenient to divide pregnancy into four stages: fertilization and implatation (<>organogenesis/embryonic stage (17-57 days), the fetogenic stage and delivery.
The principal mode of contraceptive action of progestrogens is to prevent implantation, which normally occurs 2-3 weeks after fertilization. IUCD have similar effect. Damage to the embryo before implantation results in failure of implantation and is therefore unlikely to cause fetal abnormalities.
Effects on Organogenesis/embryonic stage
The intra-uterine period between 2 weeks – 3 months is when the most serious abnormalities of fetal development can be caused by drugs. It’s during this period that the major organs are being formed. In animal studies, even one dose of a drug administered at the critical time has been shown to have a major effect.
At this stage the fetus is differentiating to form major organs and this is the critical period for teratogenesis. Teratogens cause deviations or abnormalities in the development of the embryo that are compatible with prenatal life and observable postnatally. Drugs that interfere with this process can cause gross structural defects, for example thalidomide phocomelia.
Some drugs are confirmed teratogens, but for many the evidence is inconclusive. Thalidomide was unusual in the way in which a very small dose of the drug given on only one or two occasions between the fourth and seventh weeks of pregnancy produced serious malformations. Despite its wide use it was nearly 4 years before these adverse effects were recognized.
Some drugs that are definitely teratogenic in humans.
Thalidomide Cytotoxic agents Alcohol Warfarin Retinoids Most anticonvulsants | Androgens Progestogens Diethylstilbestrol Radioisotopes Some live vaccines Lithium |
Toxicity to the formed fetus
During T2 & T3 of pregnancy, adverse effects on fetus of drugs administered to the mother are generally an exaggeration of the effects seen in the adult. Exception to this rule are the damage to tissues which are still developing e.g. teeth & bones by Tetracycline, and the impairment of brain development by Coumarin anticoagulant.
Particular care must be taken with drugs given shortly before delivery. Analgesics, e.g. Meperidine (Pethidine), and tranquillizers e.g. (Benzodiazepines) may severely impair neonatal respiration. In addition, the newborn lacks many enzyme necessary for the efficient metabolism of drugs.
Adverse effects of drugs on fetal growth and development.
- Drugs used to tread maternal hyperthyroidism can cause fetal and neonatal hypothyroidism - Tetracycline antibiotics inhibit growth of fetal bones and stain teeth - Aminoglycosides cause fetal VIIIth nerve damage - Opioids and cocaine taken regularly during pregnancy can lead to fetal drug dependency |
Delivery
Some drugs given late in pregnancy or during delivery may cause particular problems. Pethidine, regularly administered as an analgesic can cause fetal apnea (which is reversed with naloxone). Anesthetic agents given during Cesarian section may transiently depress neurological, respiratory and muscular functions. Warfarin given in late pregnancy causes a hemostasis defect in the baby and predisposes to cerebral hemorrhage during delivery.
Prescribing in Lactation:
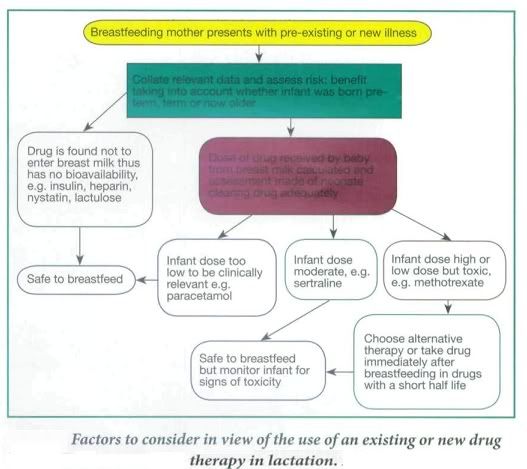
During breast feeding unintentional passage of any drug to the baby through the breast milk and the effects on the newborn must be considered. In pregnancy the mother's circulation eliminates the drug for the fetus, but once born the neonate has to clear any drug itself when the organs for drug elimination (liver and kidney) are more fragile and not functioning optimally.
It is preferable that the mother stays on stabilized medication during pregnancy and continues while breastfeeding to avoid adverse or other side-effects from changing to another drug, such as with antidepressants. However, in some cases otherwise acceptable drugs used in pregnancy are contraindicated after delivery or in breastfeeding, and prescribing an alternative, more appropriate drug would be considered at this stage. For example, methyldopa is the drug of choice for high blood pressure in pregnancy and although it is also safe in lactation, there is an increased risk of postnatal depression in women taking methyldopa; therefore it would be changed to an alternative drug postnatally.
Other factors to consider in prescribing in lactating mothers is the percentage of the drug passed into breast milk, its half-life, and whether the baby was born at term and is healthy and able to tolerate this drug, which would depend on its gestational age. Drugs taken by the mother may not be absorbed - Fybogel, Lactulose, some antibiotics such as oral vancomycin, or the antifungal, nystatin, Insulin and heparin are too large to pass into breast milk and these are usually safe. Some drugs pass in to breast milk in small amounts. In some cases this would be below therapeutic levels in the baby so would not appear to cause any adverse effects. However, a neonates organs are not fully functioning in the first few weeks of life meaning a drug could potentially accumulate over days in the neonates bloodstream, which may cause adverse side- effects. In other cases even small amounts of a drug can be harmful to the neonate, e.g. methotrexate and should therefore be avoided altogether, or the mother should consider not breastfeeding.
Specific scenarios:
- Analgesia in pregnancy and lactation:
- The drug of choice for pain relief in pregnancy is paracetamol.
- Weak opioids like codeine may be used if pain is not manageable.
- Pethidine is used as analgesia in labour as it is short- acting and its effects can be controlled.
- NSAIDs are altogether avoided because of risk to premature closure of ductus arteriosus, high risk of miscarriage due to inhibition of prostaglandin synthesis - however low dose aspirin might be used in women with PIH, umbilical placental insufficiency and clotting disorders such as lupus coagulant, Factor V Leiden and protein C deficiency, or pre-eclampsia.
- Drug of choice for pain relief during lactation is paracetamol. Tn the case of more severe pain, NSAIDs such as ibuprofen and diclofenac, are preferred. Although aspirin passes into breast milk in only small amounts, it should be avoided to prevent the risk of Reye's syndrome in children.
- Weak opiates, such as codeine and dihydrocodeine, may be used in lactation but can cause colic and constipation in the infant.
- Antihypertensives in pregnancy and lactation:
- Angiotensin II receptor antagonists, angiotensin converting enzyme (ACE) inhibitors and the beta blocker, atenolo!, are contraindicated in pregnancy because of their adverse effects on the fetus and fetal growth.
- Drugs of choice to lower high blood pressure in pregnancy are methyldopa, labetalol or nifedipine, all of which have been used safely previously.
- Some beta blockers that have low protein binding, e.g. atenolol and acebutolol, increase bioavailability of the drug in breast milk and should be avoided because of the effects of neonatal bradycardia, otherwise all anti-hypertensives are safe during lactation.
- Antidepressants in pregnancy and lactation:
- The SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline) are now most commonly prescribed to new patients as they are better tolerated than other classes of antidepressants.
- Fluoxetine has been the most extensively studied of the SSRIs in pregnancy and offers no increased risk of miscarriage, malformation or neurodevelopment to the fetus and long term.
- The SSRIs of choice for lactating women are those with the shortest half-life, i.e. tluvoxamine, paroxetine or sertraline with half lives between 17 and 26 hours. However, infants should be monitored for symptoms (owing to drug accumulation) such as excessive crying, poor sleep, irritability and colic.
- Anticonvulsants in pregnancy and lactation:
- Epilepsy in pregnancy can lead to fetal and maternal morbidity/mortality through convulsions whilst all the anticonvulsants used have been associated with teratogenic effects, for example phenytoin is associated with cleft palate and congenital heart disease.
- However, there is not doubt the benefits of good seizure control outweigh the drug-induced teratogenic risk.
- Thorough explanation to the mother, ideally before a planned pregnancy, is essential and it must be emphasized that the majority of epileptic mothers haver normal babies (>90%). (The usual risk of fetal malformation is about 2%. In epileptic mothers it is up to 10%).
- In view of the association of spina bifida with sodium valproate and carbamazepine therapy it is often recommended that the standard dose of folic acid be increased to 4-5 mg daily. Both these anticonvulsants cause hypospadias.
- As in non-pregnant epilepsy single drug therapy is preferable.
- Plasma concentration monitoring is particularly relevant for phenytoin because the decrease in plasma protein binding and the increase in hepatic metabolism may cause considerable changes in the plasma concentration of free (active.
- Antiemetics in pregnancy and lactation:
Nausea and vomiting are common in early pregnancy but are usually self-limiting and ideally should be managed with reassurance and non-drug strategies such as small frequent meals, avoiding large volumes of fluid and raising the head of the bed.
If symptoms are prolonged or severe, drug treatment may be effective.
Meclozine and cyclizine are commonly used although both have been weakly associated with an increased risk of congenital malformations.
Metoclopramide is considered safe and efficacious in labor and before anesthesia in late pregnancy but its routine use in early pregnancy cannot be recommended because of lack of controlled data, and a significant incidence of dystonic reactions in young women.
Drugs for Acid-peptic and constipation during pregnancy and lactation:
The high incidence of dyspepsia due to gastro-esophageal reflux in the second and third trimesters is probably related to the reduction in lower esophageal sphincter pressure.
Non-drug treatment - reassurance, small frequent meals and advice on posture - should be pursued in the first instance particularly in the first trimester. Fortunately most cases occur later in pregnancy when non-absorbable antacids such as alginates should be used.
In late pregnancy metoclopramide is particularly effective as it increases lower esophageal sphincter pressure.
There are inadequate safety data on the use of H2-receptor blockers and omeprazole in pregnancy.
- Sucralfate has been recommended for use in pregnancy in the USA and is rational as it is not systemically absorbed.
- Misoprostol, a prostaglandin which stimulates the uterus, is contraindicated because it causes abortion.
Constipation should be managed with dietary advice. Stimulant laxatives may be uterotonic and should be avoided if possible.
- Anticoagulants in pregnancy and lactation:
Warfarin has been associated with nasal hypoplasia and chondrodysplasia when given in the first trimester and CNS abnormalities after administration in later pregnancy, as well as a high incidence of hemorrhagic complications towards the end of pregnancy. Neonatal hemorrhage is difficult to prevent because of the immature enzymes in fetal liver and low stores of vitamin K.
Heparin, which does not cross the placenta, is the anticoagulant of choice in pregnancy although chronic use can cause maternal osteoporosis.
Women on long-term oral anticoagulants should be warned that these drugs are likely to affect the fetus in early pregnancy.
Subcutaneous heparin (usually self-administered) must be substituted for warfarin as soon as possible, well before the critical period of 6-9 weeks´ gestation. Subcutaneous heparin can be continued throughout pregnancy but due to the risk of maternal osteoporosis and thrombocytopenia warfarin may be considered as an alternative during the second trimester changing back to heparin at 36 weeks.
Patients with prosthetic heart values present a special problem: in these patients in spite of the risk to the fetus warfarin is often given up to 36 weeks.
The prothrombin time/international normalized ratio (INR) (warfarin) or APTT (activated partial thromboplastin time) (heparin) should be monitored closely.
Hormones in pregnancy and lactation:
- Progestogens, particularly synthetic ones, can masculinize the female fetus. There is no evidence that this occurs with the small amount of progestogen (or estrogen) in the oral contraceptive: the risk applies to large doses.
- Corticosteroids do not appear to give rise to any serious problems when given via inhalation or in short courses. Transient suppression of the fetal hypotlalamic-pituitary-adrenal axis has been reported. Rarely cleft palate and congenital cataract have been linked with steroids in pregnancy but the benefit of treatment usually outweighs any such risk.
Conclusion:
- A detailed risk:benefit assessment of drug therapy in pregnancy should be conducted.
- There are altered pharmacokinetics in the pregnant woman affecting drug metabolism and elimination.
- It is important to consider the trimester of pregnancy when prescribing.
- Known teratogenic drugs must be avoided in pregnancy.
- Some acceptable drugs used in pregnancy may be contraindicated after delivery or in breastfeeding and vice versa; the two should be considered independently.
- Drug prescribing in the breastfeeding mother depends on the percentage of drug passed in to breast milk, the drug half-life, and whether the baby was born at term and is healthy.
Essential Drug Concept
A growing number of pharmaceutical products are available on the world market and there has been an increase, both in the consumption of drugs, and in expenditure on them. But in spite of this, many people throughout the world cannot obtain the drugs they need. There are also many people who do have access to drugs but who do not get the right drug, in the right dosage, when they need it. The essential drugs concept was developed in response to these problems and continues to be central to policies and strategies that aim to address them. The essential drugs concept is central to the development of national drug policy.
The World Health Organisation (WHO) defines essential drugs as -
Essential drugs are those that satisfy the health care needs of the majority of the population; they should therefore be available at all times in adequate amounts and in the appropriate dosage forms.
The first WHO model list of essential drugs was published in 1975.Since 1977, WHO has promoted the use of limited Essential Drug Lists (EDL) as part of Essential Drug Concept. Initially drugs were selected on the basis of experience. Recently WHO has begun to advocate a change towards an “evidence-based” approach. The Indian government published its first National Essential Drugs list (EDL) in 1996, and some state governments have also adopted an EDL. It is believed that only about 250 drugs are essential to treat the majority of diseases in a country. Selecting some drugs as part of the EDL does not lower the importance of other drugs - a list of essential drugs is a positive list, mainly intended to define priority drugs for public procurement and reimbursement, and for training of health personnel

Selection of Drugs in EDL
Correct selection and purchase of pharmaceuticals aims to improve the affordability and quality of health care to the population. Implementation of the ED concept starts with selection of drugs to be on the national EDLs. Selection is a complex issue and the committee considers multiple factors such as the available human and financial resources, disease prevalence, health indicators and demographic situation. The primary criteria for inclusion are accurate clinical data on safety and efficacy of the drug, its availability, cost and cost-effectiveness. Ideally the selection process should be consultative and transparent; the criteria should be explicit and linked to evidence-based clinical guidelines.
- Pattern of prevalent diseases
- Drugs required for national programs given priority
- Treatment facilities available
- Training and experience of available personnel
- Safety, Efficacy, Cost and Ease of administration
- Generics are cheaper
- Newer is not always better
- Storage conditions
- Good manufacturing processes
- Patient acceptability
- Medicine Surveys
- To measure effects of information/regulation on drug usage pattern
- To study absolute/relative efficacy and safety
- To indicate overuse/under use or misuse of drug/drug classes
Generally fixed-dose combinations are not encouraged in the EDL except where clear benefits are known (E.g. Co-Trimoxazole, Oral Contraceptives). The selection of drugs in the EDL is a continuing process and review and revisions must be made as and when new advances take place in pharmacological and pharmaceutical knowledge.
National Drug Policy of India
With a population of nearly 100 crores, India accounts for 16 per cent of the global population. Sizeable population lives below the poverty line and 48 per cent of the people are illiterate. India was among the first few countries to attempt at a National drug policy. This has resulted in the establishment of a pharmaceutical industry, the development of technological capabilities in pharmaceuticals and price control of selected essential drugs.
In the initial 3 decades after independence, public sector production units were set up to produce drugs such as antibiotics etc. which the private sector/ foreign owned companies were reluctant to produce. Indian Patents Act, 1970 disallowed pharmaceutical product patents and limited duration of process patents to 5-7 years - helping in growth of domestic manufacturers. The Drug Control General India (DCGI) was created on similar lines to the Food and Drug Administrations (FDAs).
In the early 1990's liberalization policies led to a two to threefold rise in prices of many important drugs. A National Pharmaceutical Pricing Authority (NPPA) was formed in 1996 to ensure price stability and availability of commonly used medicine. To ensure international standards Schedule M of the Drugs and Cosmetic Acts, 1945 was amended in 2001. In 2003 the Essential Drugs List was amended/ reviewed for the first time after it was created in 1996. In 2004, India became a signatory to the GATT - Uruguay round of International Trade Agreement - hence patents are back for 20 year durations and cost of many drugs are bound to rise.
There are a total of 354 drugs in the National List of Essential Medicines (NLEM), which are adequate to take care of the majority of the health needs of the population. But when we analyze the sales of top 300 brands only 38% of brands are of the drugs mentioned in the NLEM. The other 62% brands comprise drugs that are higher priced alternatives without a clear therapeutic advantage and many are unnecessary, irrational and even hazardous.This indicates that contrary to what is advocated by WHO & as adopted in our national health policy, the drugs which are most useful & cost effective for the treatment are not being prescribed & the non costlier & irrational drugs are sold more often.
Rational drug use has not been integrated into government policy, and non-essential drugs and irrational drug combinations continue to be approved for marketing. Ethical promotion of drug advertising, funding for drug information centres, drug utilisation research, patient education in medication use should also be a part of any national drug policy; which are sadly, missing in the National Drug Policy of India.
Rational Drug Use
Rational drug use (RDU) is often defined as
"the use of an appropriate, efficacious, safe and cost-effective drug for the correct indication in the right dose and formulation at correct intervals and duration."
This involves adoption of the essential drug concept & development and usage of evidence based clinical guidelines. Other vital strategies to promote RDU include unbiased and independent drug information and continuing medical education.
Types of Irrational use of Medicines:
There are several types of irrational use of medicines -
- Use of medicine when no drug therapy is needed (antibiotics in viral upper respiratory tract infections)
- Use of wrong medication for conditions requiring specific drug therapy (Fixed drug combination of Norfloxacin and Metronidazole in diarrhea)
- Use of medicine of doubtful or unproven efficacy (Vitamins in the absence of deficiency)
- Use of medicine of uncertain safety (ayurvedic bhasmas)
- Failure to prescribe available, safe and effective agents (Under use of antidepressants or antihypertensives)
- Incorrect administration, dosages or duration.
- Use of too many drugs per patient (Polypharmacy)
- Use of injections when oral formulations available
- Inappropriate self-medication, often of prescription only medicine
- Incorrect treatment of disorders
- Poor compliance
Reasons for Irrational drug use:
- Misinformation, misleading beliefs or inability to communicate symptoms correctly on part of the patient.
- Wants quick response - believes injections work faster
- Unhappy if no medicine prescribed
- compliance is big problem - once symptoms subside, stops treatment
- Adherence of patients to a treatment program is necessary for the success of the program. Non-adherence or non compliance results from factors related to the drug, the patient, the prescriber and the environment.
- Almost all drugs available OTC
- Lack of accurate and correct information of newer agents, workload and pressure to prescribe on part of the prescribers
- Poor diagnostic support - empirical treatment
- No evidence based guidelines readily available
- Continuing education programmes
- Poor medical facilities and supply of drugs
- Vaccines and many other drugs not stored at required temparature
- Over 80,000 formulations in the country, many doubtful value
- Inadequate drug regulation -
- Process for marketing approval of new drugs
- Controls on OTC drugs
- More effective definition of prescriber
- Quality assurance of products
- good drug distribution systems
- availability and price control of essential drugs
- Unbiased drug information
- Unethical promotion of drugs
Impact of Irrational Drug use:
- Poor quality of health care delivery - Increased morbidity and mortality
- Waste of scant resources on using wrong medicine
- Irrational drug use leads to ineffective and unsafe treatment, worsening or prolonging of illness and adverse drug reactions.
- It also unnecessarily adds to the economic burden of the patients, the government or the insurance system.
- The widespread antibiotic resistance is also partly a result of irrational use of antibiotics.
Guidelines for Rational Drug Use:
- Define the patient's problem - define the therapeutic objective
- Use drug only when indicated and when potential benefits outweigh any risks
- seriousness of the problem to be treated
- efficacy of the drug to be used
- severity and incidence of possible ADRs
- safety and efficacy of alternative drugs if any
- e.g., phenylbutazone, commonly used in the treatment of osteoarthritis in the past, because of its high benefit-to-risk ratio, is currently replaced with safer drugs.
- Assessment of benefit-to-risk ratio may not always be easy. The following examples show this problem:
- The benefit of adding digoxin to a diuretic and vasodilator vis- à-vis the risk of its toxicity in the treatment of congestive cardiac failure. This might depend on the cause of the heart failure, patient compliance, renal function and ease of monitoring of plasma digoxin concentration
- The benefit from a course of antibiotic in treating urinary tract infection in two months pregnant as compared to the risk of treatment to the fetus. Here the risk of teratogenesis needs to be compared to the risk of renal damage to the mother as a result of untreated infection
- Choose a drug of proven efficacy and safety, which is suitable and affordable for the patient
- The use of International Non Proprietary Names (INN) or generic names of drugs in prescribing is an essential component of good prescribing practice. This is because generic drugs are less costly, an important factor in our country, and for a generic prescription any suitable product can be dispensed hence avoiding delay while looking for a specific brand.
- Avoid same class drug
- Choose suitable dose
- Route of administration: e.g.,
- Crystalline penicillin is given by intravenous route for severe infections; Intramuscular benzathine penicillin monthly or oral penicillin daily can be given for rheumatic fever prophylaxis.
- Beta agonists like salbutamol are given through nebulizers or inhalers in acute asthmatic attack for fast action.
- Formulations : e.g.,
- oral formulations include tablets, capsules, granules, elixirs, suspension; injection formulations include lyophilized powders, solution.
- For acute pain, prescribe soluble buffered aspirin because of fast action
- For chronic rheumatic disease, prescribe enteric-coated formulation
- Dosage regime(dose) frequency/timing of administration: the following need to be considered
- Kinetic variability: for poor absorption prescribe larger dose or prescribe another drug/route
- Dynamic variability: for less effect, prescribe larger dose
- Characteristics of the patient: tailor the dose according to body weight and other factors while prescribing
- Characteristic of the disease: e.g., dose of codeine used to suppress cough is lower than that required to relieve pain
- Choosing a dosage regimen by
- referring to a reliable source of information
- considering dose related toxicity
- deciding the initial dose: when there is no guide, start with low dose and increase gradually (ACE inhibitors, levodopa); for some drugs to increase the dose may be necessary because of tolerance (opiates); sometimes starting with a loading dose before giving a maintenance dose is required (digoxin); in some cases to start with high dose and reduce gradually might be required (corticosteroids)
- Considering kinetic factors which may alter dosage requirements: e.g., impaired renal function
- Considering dose response relationship for the patients: e.g., higher dose of insulin for ketoacidosis, and lower dose of antipsychotic drugs in treatment naive patients
- Considering other patient characteristics like age, sex weight, etc
- Frequency of drug administration:
- usually fixed for a given formulation;
- sometimes may be altered (e.g., splitting the dose of spiranolactone - in two to avoid GI irritation),
- frequency of nitroglycerin administration depends on the frequency of symptoms
- Timing of drug administration: The following are examples where timing is very important
- To minimize ADR: tricyclic antidepressants to avoid dry mouth and drowsiness better to take it at bed time; potent diuretics better be given in the morning to avoid disturbance during the night
- Timing of symptoms: e.g., anginal attack, use of antacids
- Timing in relation to meals: penicillin to be administered before meal; aspirin to be administered with meal
- Inform the patient - dose, duration, adverse effects if any
- Monitor treatment
- If drug ineffective find reason, any changes needed or monitoring required
- Whether to continue drug or stop
- Depends on the nature of the disease or symptom: e.g., A single dose of aspirin for headache;Insulin for chronic therapy; treatment with H2 blockers require six weeks as healing occurs with in this period, but longer periods of nightly administration may be required to prevent recurrence.
- Duration of treatment of infection depends on: The infecting organisms , Site of infection, Response to treatment, Dosage of antibiotics
- Informing and persuading prescriber - from students to practicing clinicians and community (teaching, seminars, face to face talks, distribution of printed matter, television etc)
The first step in rational treatment is defining the patient’s problem, which is making a correct diagnosis. Once a diagnosis is made, one has to specify his/her therapeutic objective, what the physician wants to achieve with the treatment to be applied. Based on the therapeutic objective, one chooses a treatment of proven efficacy, safety, suitability and affordable cost from different alternatives.
The treatment may consist only of giving patients’ advice and information about their illness, non-drug therapy, treatment with drug, or a combination of these. For every diagnosis you make, the treatment plan you choose will be your P (personal)-treatment.
When for a certain diagnosis your P-treatment consists of drug treatment, you have got to choose a drug(s), on the basis of efficacy, safety, suitability, cost and availability. This will be your P - (personal) drug. P-drugs are list of drugs every subscriber got to choose for a particular problem based on the National Essential Drugs list and the Standard Treatment Guideline (STG). The prescriber, by choosing P-drugs which he prescribes regularly, becomes familiar with their effects and side effects. The P-drug concept is more than just the name of a pharmacological substance, it also includes the dosage form, dosage schedule and duration of treatment.
Drug Therapy in Liver Disease
Introduction
The liver is the principal organ of metabolism in the body although other sites are involved such as the gut wall, kidney, skin and lungs. Drug metabolism, by means of enzyme reactions in the liver, is the body's main method of metabolizing drugs. Drug molecules are converted into more polar compounds, which aid their elimination. Generally, metabolism results in the loss of pharmacological activity because transport to the site of action is limited due to reduced lipid solubility, or because the molecule is no longer able to attach to its receptor site. However, in some circumstances drugs are metabolized to more active forms, for example the conversion of codeine to morphine, primidone to phenobarbitone and amitriptyline to nortriptyline.
Concentrations of enzymes involved in both phase I and II reactions vary significantly between individuals with normal hepatic function and even more so between the healthy population and those with hepatic impairment.
Chronic liver disease is more predictably associated with impaired metabolism of drugs than acute liver dysfunction. However, in cases of severe acute liver failure, the capacity to metabolize the drug may be significantly impaired.
In the chronic state, cirrhosis of any aetiology, viral hepatitis and hepatoma can decrease drug metabolism. In moderate to severe liver dysfunction, rates of drug metabolism may be reduced by as much as 50%. The mechanism is thought to be due to spatial separation of blood from the hepatocyte by fibrosis along the hepatic sinusoids.
The use of certain drugs in patients with cirrhosis occasionally increases the risk of hepatic decompensation. An example of this is the increased risk of hepatic encephalopathy in some patients who receive pegylated interferon alfa-2a in combination with ribavirin for the treatment of chronic active hepatitis related to the hepatitis C virus. In addition, co-infection with hepatitis B or C virus, even in the absence of cirrhosis, increases the risk of hepatotoxicity from antiretroviral therapy in patients with coexistent HIV infection.
In the presence of chronic liver disease, there is potential for changing the systemic availability of high extraction drugs, thereby affecting plasma concentrations. A potential consequence of liver disease is the development of portosystemic shunts that may carry a drug absorbed from the gut through the mesenteric veins directly into the systemic circulation. As such, oral treatment with high hepatic clearance drugs such as morphine or propranolol can lead to high plasma concentrations and an increased risk of adverse effects.
Liver damage can also affect drugs with low hepatic clearance. For instance, the effect of warfarin, which has a low extraction ratio, is increased due to the reduced production of vitamin K-dependent clotting factors.
The pharmacokinetic interaction between alcohol and drugs is more complex. An acute ingestion of alcohol may inhibit a drug's metabolism by competing with the drug for the same set of metabolising enzymes. Conversely, hepatic enzyme induction may occur with chronic excessive alcohol ingestion via CYP2E1 resulting in increased clearance of certain drugs (for example phenytoin, benzodiazepines). After these enzymes have been induced, they remain so in the absence of alcohol for several weeks after cessation of drinking. In addition, some enzymes induced by chronic alcohol consumption transform some drugs (for example paracetamol) into toxic compounds that can damage the liver.
In the presence of cholestatic jaundice, drugs and their active metabolites that are dependent on biliary excretion for clearance will have impaired elimination. Further impairment will occur if the compound is excreted as a glucuronide and is subject to enterohepatic circulation.
High extraction ratio | Low extraction ratio |
Antidepressants Chlorpromazine/haloperidol Calcium channel blockers Morphine Glyceryl trinitrates Levodopa Propranolol |
Non-steroidal anti-inflammatory drugs Diazepam Carbamazepine Phenytoin Warfarin |
Evaluating Hepatic Function
It is also important to look for signs of acute or chronic liver disease such as the presence of jaundice, spider naevi, palmar erythema, ascites, abdominal distention, hepatomegaly, splenomegaly and caput medusa. If there is clinical evidence of liver disease, further investigation is required. This includes liver function tests and an ultrasound of the abdomen. A portal vein Doppler study is also recommended to assess for the presence of portal hypertension. A slowing or reversal of portal vein blood flow indicates portal hypertension which may be related to either liver cirrhosis or portal vein thrombosis.
In renal disease, serum creatinine concentration and the glomerular filtration rate provide a reasonable guide to drug dosage requirements. In contrast, there is no single test that measures liver function so a reliable prediction of pharmacokinetics is not possible. Some evaluation of hepatic function is possible by assessing serum albumin and bilirubin, and prothrombin time. However, these parameters are not directly related to drug clearance. Although not directly correlated with liver dysfunction, elevated liver enzymes may raise the suspicion of hepatic impairment requiring further investigation.
The Child-Turcotte score was designed to estimate the operative risk of an alcoholic patient with cirrhosis. The parameters used include serum concentrations of bilirubin and albumin, prothrombin time, nutritional status and ascites. These parameters were modified to substitute degree of encephalopathy for nutritional status and then became known as the Child-Pugh classification.The grades A, B and C may also be a useful indicator of an individual's ability to effectively metabolize a drug. An alternative method for assessing liver dysfunction is the Model for End-Stage Liver Disease (MELD) score. This may be a more accurate method but is less accessible to most clinicians because it involves calculating the score.
Parameter | Points assigned = 1 | Points assigned = 2 | Points assigned = 3 |
Ascites | Absent | Slight | Moderate |
Bilirubin, micromol/L | <11 | 11–45 | >45 |
Albumin, g/L | >35 | 28–35 | <28 |
Prothrombin time – seconds over control | <4 <1.7 | 4–6 | >6 |
Encephalopathy | None | Grade 1–2 | Grade 3–4 |
Total score of 5–6 is grade A or well compensated disease (1 and 2 year survivals are 100% and 85%) Total score of 7–9 is grade B or disease with significant functional compromise (1 and 2 year survivals are 80% and 60%) Total score of 10–15 is grade C or decompensated liver disease (1 and 2 year survivals are 45% and 35%) Depending on hepatic clearance and the therapeutic index of the drug, dose adjustments or drug avoidance may be required in grades B or C chronic liver disease. | |||
Evaluating the drug in question
Drugs with a narrow therapeutic range that are extensively metabolised by the liver (that is, greater than 20% of their total elimination) should either be avoided altogether (e.g. pethidine) or used with extreme caution (e.g. morphine, theophylline) in patients with significant liver disease.
Drugs with a wide therapeutic range which also undergo extensive hepatic metabolism should be used with caution. In particular, the dosing interval should be increased or the total dose reduced (e.g. carvedilol).
If hepatic elimination is limited (that is, accounting for less than 20% of total elimination), then the therapeutic range of the compound should be reviewed. If the drug has a wide therapeutic index, then the likelihood of an adverse effect related to hepatic impairment is low. However, if the drug has a narrow therapeutic index, then caution should be exercised as significant hepatic impairment may have a clinically relevant effect on the pharmacokinetics (e.g. lamotrigine).
If greater than 90% of the compound is excreted unchanged in the urine, then hepatic impairment is unlikely to play a significant role in the accumulation of the drug and therefore toxicity.
Factors to consider when prescribing drugs dependent on hepatic elimination
- Ascertain how much the drug depends on hepatic metabolism for its elimination from the body.
- Determine the degree of hepatic impairment using the Child-Pugh classification, hepatic enzyme levels and possibly an ultrasound of the liver with portal vein Doppler study.
- If there is doubt about the degree of hepatic impairment or the drug has a narrow therapeutic index (that is, the upper dose range for efficacy is close to the lower concentration range of toxicity), then lower the recommended starting dose by approximately 50%, and titrate to effect under careful supervision – 'start low and go slow'.
- Determine possible interactions between the new drug and any drugs the patient is already taking.
Reference: Sloss A, KublerP. Prescribing in liver disease; Aust Prescr 2009: 32:32-5
Herbal Products Drug Interactions
Introduction:
Until 150 years ago, all medicines were derived from natural substances. Most of these early medicines were described under the broad heading ‘herbs’ although that term was misleading. Even though people often think of herbs as plant or plant-derived materials, several commonly used items were obtained from animals and minerals. Further, the term ‘herbs’ suggests something that is beneficial and has little potential for harm. A recent survey shows that 18% of adults in the United States use prescription drugs concurrently with herbal or vitamin products, placing an estimated 15 million people at risk of potential drug interactions. It is quite common for a patient to seek herbal treatment while taking several prescription medications and safety has become a major issue. The manufacturers of these herbal products are not required to submit proof of safety and efficacy to the USFDA before marketing. For this reason, the adverse effects and interactions associated with herbal remedies are largely unknown. Concurrent use of herbs may mimic, magnify or oppose the effects of drugs. The possibility of drug-herb interactions is very high. The outcome of the interactions may lead to adverse effects.
Types of herbal interactions:
Herbal interactions can be classified into pharmacokinetic and pharmacodynamic interactions as that of modern medicines. The former involves mainly the Cytochrome P450 drug metabolizing enzymes in the liver microsomes. For example, the constituent of garlic inhibits the activity of various CYP isoforms, including CYP3A4 in vitro, Silibinin the major constituent of milk thistle inhibits CYP3A4 and CYP2C9 activities in vitro. Peppermint oil and menthol (a constituent of peppermint oil) inhibits CYP3A4 activity in vitro. The pharmacodynamic interactions can be predicted if the phytochemical ingredients of a herb and the pharmacology of the interacting drugs are known.
The most common example for this type is the increased risk of bleeding due to warfarin when taken with Garlic. Some of the clinically significant drug-herb interactions are summarized in the table below.
Herbal drug | Indication | Interacting drugs | Outcomes |
Capsicum (Capsicum annum) | Carminative, Antirheumatic and in Neuralgia | ACE inhibitors, Theophylline, Sedatives, Antidepressants | Increases drug absorption and effect; possibility of cough with ACE inhibitors |
Garlic (Allium sativum) | Antihyperlipidemic and antihypertensive
| Aspirin, Clopidogrel Dipyridamole, Warfarin Cyclosporine, Saquinavir | Risk of bleeding with anticoagulants. Decreased effect of immunosuppressants and protease inhibitors |
Ginger (Zindiber officinale) | Antiemetic and antivertigo antivertigo | Aspirin, Ticlopidine, Clopidogrel, Dipyridamole, Warfarin, H2-blockers and Proton Pump Inhibitors | Increased risk of bleeding. May counteract antiulcer medications. |
Ginkgo (Ginkgo biloba) | Increasing blood circulation, oxygenation and for improving memory and mental alertness | Aspirin, Ticlopidine, Clopidogrel, Dipyridamole, Warfarin, Antidepressants, Antipsychotics, Insulin | May increase the antiplatelet and anticoagulant effect and increased risk of bleeding. Increased risk of seizures with antipsychotics and affects insulin levels. |
St.John's wort (Hypericum perforatum) | Depression, enlarged prostate and urinary inflammations | Antidepressants, Protease inhibitors, Non-nucleoside reverse transcriptase inhibitors, Digoxin, Theophylline, Cyclosporine, Oral Contraceptive Pills (OCPs) | Known to cause serotonin syndrom. May reduce the effectiveness of OCPs and bioavailability of digoxin, theophylline and cyclosporine |
Shankapuspi (Canscora decussata) | Nervine tonic | Pheytoin | Reduced Anti-epileptic activity |
Aloe (Aloe vera) | Carminative, Purgative | Digoxine, Thiazide | Increased cardiac toxicity due to electrolyte imbalance. |
Conclusion:
Historically, herbs and drugs have been two very different treatment modalities, which have rarely been used together. The line that separates herbs and drugs, however, has been blurred in recent decades with the increased accessibility to the lay public of different treatment modalities. Even though herbal products are available without prescription, medical guidance is necessary because of the adverse effects of these products and the potential for drug interaction.
The solution to this situation lies in the understanding of drug-drug and drug-herb interaction in detail with scientific evidence. With understanding of these mechanisms, one can recognize potential interactions and take proper steps to prevent their occurrence.